Fibromyalgia: Centralized Pain Processing and Neuroimaging
Fibromyalgia is a chronic, widespread pain disorder that affects a substantial portion of patients. Prevalence in the general population is 2%−4%, with women being diagnosed more frequently than men (12). A systematic review and meta-analysis reported fibromyalgia prevalence to be much higher in clinical populations (15.2% of rheumatology/internal medicine patients, 14.8% of patients with type 2 diabetes, and 12.9% of patients with irritable bowel syndrome) (13). A retrospective cohort study of primary care patient encounters (academic medical center) identified 4.6% of patients with fibromyalgia (14). It was associated with a higher prevalence of comorbid conditions. Symptoms consistent with fibromyalgia have been documented since the early 19th century; however, the disorder was formally named fibromyalgia syndrome in 1976 (15). Fibromyalgia was initially classified as a rheumatological disorder (15). In recent decades, multiple lines of research have shifted focus from a peripheral to a central neurobiological basis (8, 16).
Identifying a common language and classification to diagnose and treat fibromyalgia has been challenging, because patients may first seek care from different disciplines (e.g., rheumatology, neurology, psychology, psychiatry, and family medicine) with unique perspectives and terminologies (12, 17). Fibromyalgia, similar to other medically unexplained pain syndromes, may be classified in numerous ways (e.g., functional somatic syndrome, chronic widespread pain syndrome, persistent somatoform pain disorder, somatic symptom disorder, affective spectrum condition, and central sensitivity syndrome) (12, 18). There remains an ongoing debate as to whether fibromyalgia should be considered a distinct disorder or grouped with other disorders that have overlapping symptomology (e.g., irritable bowel syndrome, chronic fatigue syndrome, and chronic pelvic pain syndromes) into a chronic widespread pain syndrome spectrum (15, 19, 20).
Two international workgroups have recently released new classification criteria for chronic pain conditions (21, 22). The International Association for the Study of Pain (in collaboration with World Health Organization) developed new ICD-11 codes that separate chronic pain conditions by whether or not the pain is secondary to another condition (e.g., osteoarthritis, diabetes, and cancer) (22).The new designation of chronic primary pain syndromes includes fibromyalgia in the chronic widespread pain category. The American Pain Society (in collaboration with multiple other groups) led the efforts to improve diagnostic classification criteria for chronic pain conditions (21). The workgroup determined that the core diagnostic criteria (dimension 1) for fibromyalgia are pain of at least 3 months duration occurring in at least six body sites (using the nine-site Manikin) that is accompanied by fatigue (physical or mental) or sleep disturbances judged to be of at least moderate severity by a clinician (23). Other common features (dimension 2) include tenderness (widespread heightened sensitivity to pressure), executive functioning deficits (disorganized/slow thinking, difficulty concentrating, forgetfulness), and sensory intolerance (heightened sensitivity to lights, sounds, odors, or cold). Common comorbidities (dimension 3) include several psychiatric conditions (major mood disorders, anxiety disorders, substance use disorders) (23).
Identified risk and vulnerability factors for developing fibromyalgia include female gender, middle to older age, limited physical activity, genetic factors, premorbid psychosocial stress, and co-occurring mental health symptoms (24–26). Recent research suggests that up to 50% of risk may be attributable to genetics, specifically the expression of hypomethylated DNA and microRNAs (27). A recent systematic review of studies examining associations between traumatic life events and development of fibromyalgia supported associations between both physically traumatic events (e.g., physical injury, surgery) and psychologically traumatic events (e.g., sexual or physical abuse, emotional neglect) (26). There is further evidence that traumatic experiences and chronic stress may result in epigenetic alterations of genes associated with DNA repair (27). Psychosocial resilience and protective factors include strong social support, active coping strategies, acceptance, psychological flexibility, and increased self-efficacy. These have been shown to be beneficial in reducing experiential pain and improving general wellbeing (28).
Diagnostically, medically unexplained chronic pain is closely related to somatic symptom disorders, as identified in DSM-5 (19, 29, 30). However, there is evidence that individuals with somatic symptom disorders and fibromyalgia present with differing symptomology and behaviors. A study that separated fibromyalgia patients by whether they fulfilled DSM-5 criteria for a somatic symptom disorders reported that the study groups differed in the level of self-reported symptoms (e.g., symptom burden, psychological distress, pain catastrophizing, and pain-related disability) (31). In contrast, the study groups did not differ on objective measures of health care utilization (e.g., the number of health care visits in the previous 6 months, number of hospital stays for chronic pain, and number of doctors consulted) or disability (e.g., sick leave days, applying for disability pension). Although psychiatric symptoms were prevalent in both groups, a higher proportion of the fibromyalgia patients meeting somatic symptom disorders criteria also met criteria for an anxiety or depressive disorder (95% versus 72%). The authors commented that disproportionate and persistent worry about health symptoms may be better attributed to the comorbid depressive or anxiety disorders than to fibromyalgia (31). As noted in several recent reviews, fibromyalgia is more accurately classified as having both physical and psychological dimensions rather than categorizing it as either a physical disorder or a psychiatric disorder (32–35).
A recent prospective population-based cohort study reported a bidirectional relationship between chronic pain conditions and psychiatric disorders, as developing one increased the risk for developing the other (36). The incidence rate ratio (IRR) for receiving a fibromyalgia diagnosis after developing a psychiatric disorder (compared with not having a psychiatric disorder) was 5.54 (95% CI=4.99–6.16). The IRR for receiving a psychiatric diagnosis after developing fibromyalgia (compared with not having fibromyalgia) was 4.05 (95% CI=3.58–4.59). As noted by the authors, these results suggest shared biopsychosocial vulnerabilities between persistent pain and psychiatric disorders (36).
Centrally mediated amplification of pain (central sensitization), an adaptive response to limit further injury (sickness behavior), normally subsides with healing (37, 38). Quantitative sensory testing (QST) is a standardized methodology for assessing reactions (self-report measures, physiological responses) to delivery of quantified peripheral stimuli. QST provides several metrics that are used to identify changes in perception or processing of peripherally delivered stimuli that indicate presence of central sensitization (20, 37, 39). These include pain resulting from typically nonpainful stimuli (allodynia), increased sensitivity to painful stimuli (hyperalgesia), enhanced progressive increase in pain with repeated exposure (temporal summation, wind up), and impaired conditioned pain modulation (prior conditioning stimulus normally decreases perceived pain to painful stimulus). Central sensitization has been demonstrated in both secondary chronic pain conditions (pain is evoked or exacerbated by peripheral stimuli such as inflammation or neuropathy) and in primary chronic pain conditions (no external triggers identified, such as fibromyalgia) (20, 38–40).
Many studies utilizing QST have demonstrated presence of central sensitization in patients with fibromyalgia (20, 39, 40). A meta-analysis of studies comparing fibromyalgia and healthy control groups on QST metrics confirmed enhanced temporal summation and impaired conditioned pain modulation in fibromyalgia patients (41). A few studies have utilized QST in combination with clinical measures to evaluate treatment-related changes in patients with fibromyalgia. A randomized-blinded clinical trial comparing amitriptyline and melatonin (alone and in combination) reported improvement in conditioned pain modulation and slightly better symptom reduction in the groups receiving melatonin (42). A small study that administered QST prior to and during treatment with pregabalin reported gradual improvements in pain thresholds and conditioned pain modulation as well as reductions in other symptoms (e.g., Fibromyalgia Impact Questionnaire, 12-item short form health survey) (43). A pilot study of mindful yoga for fibromyalgia reported improved pain tolerance and decreased pain after sensations accompanied by clinically meaningful improvements on symptom severity, pain, and pain catastrophizing (44). Overall, these studies indicate the presence of central sensitization prior to treatment initiation in patients with fibromyalgia that is as least partially normalized following treatment.
MRI studies have used voxel-based morphometry to identify differences in regional brain volumes between fibromyalgia and healthy control groups (3, 16, 45). Two meta-analyses of such studies reported similar areas of decreased volume in anterior and posterior cingulate cortices (Figure 1) (1, 2). Of these meta-analyses, one also identified other areas of decreased (parahippocampal/fusiform cortex) and increased (cerebellum) volume in fibromyalgia (2). Single studies have reported differences in multiple other regions (thalamus, pons, precuneus, and basal ganglia) (1, 2, 16, 45). A longitudinal study in fibromyalgia patients with insomnia assessed regional cortical thickness in areas previously identified as altered in either condition before and after treatment (46). Finding support the potential reversibility of some of these differences.
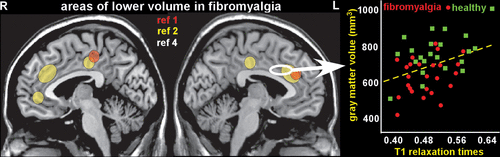
FIGURE 1. Two meta-analyses of MRI studies that used voxel-based morphometry to compare regional brain volumes between patients with fibromyalgia and healthy individuals reported similar areas (yellow, orange) of decreased volume in anterior and posterior cingulate cortices (1, 2). Very little is known regarding the types of tissue changes that underlie identified volumetric differences (3). One study combined multiple imaging approaches to assess contributions of neuronal density and water content in areas that differed in volume between fibromyalgia and healthy groups (4). Decreased water content (indicated by decreased T1 relaxation times) accounted for a substantial proportion of variance in areas with lower volume in the fibromyalgia group. Graphed are data from the region of interest marked on the MRI (white outline), color coded by group. Neuronal density did not differ, suggesting absence of neurodegeneration in fibromyalgia (4).
Very little is known regarding the types of tissue changes that underlie identified volumetric differences (3). One study combined multiple imaging approaches with hierarchical multiple regression analyses to assess contributions of neuronal density and water content in areas that differed in volume between fibromyalgia and healthy control groups (4). [18F] flumazenil positron emission tomography (PET) binding provided a surrogate measure for neuronal density. This ligand binds to the benzodiazepine site on the GABAA receptor complex. MRI T1 relaxation time measurement provided a surrogate measure for water content. GABAA receptor binding did not differ between the groups in any region of interest (ROI), suggesting absence of neurodegeneration in fibromyalgia. Decreased water content accounted for a substantial proportion of variance in ROIs with lower volume in the fibromyalgia group. Both GABAA receptor binding and increased water content contributed to variance in ROIs of higher volume in the fibromyalgia group. As noted by the authors, these results might indicate presence of neuroinflammatory edema (4). A multisite study utilized [11C] PBR28 PET to compare translocator protein (TSPO) binding (glial marker) between fibromyalgia and healthy control groups (47). This study identified multiple cortical areas (primarily in lateral and medial frontal and parietal lobes) with higher binding in the fibromyalgia group, indicating presence of glial activation (47). Exploratory analyses indicated that the only clinical variable that correlated with binding was fatigue. Fibromyalgia patients with higher levels of fatigue had higher TSPO binding in anterior and middle/posterior cingulate cortices. [11C]-l-deprenyl-d2 PET, considered a marker specific to astroglia, did not differ between the groups. Thus, areas of increased TSPO binding likely indicate presence of microglial activation in fibromyalgia. As discussed by the authors, the functional significance of these changes is presently undetermined (i.e., adaptive or maladaptive microglial responses) (47).
Meta-analyses of functional MRI (fMRI) studies in which painful stimuli were administered have reported generally similar patterns of activations in healthy individuals and in patients with chronic pain conditions (5, 48, 49). One meta-analysis found that although findings varied considerably across individual fMRI studies (i.e., insula was the most frequently reported area, yet was reported in <70% of studies), the frequency of an area being reported was similar for both groups (Figure 2) (5). In contrast, the other meta-analysis identified multiple areas more likely to be activated in either healthy individuals or patients with chronic pain (48). A third meta-analysis combined studies using different functional imaging techniques (fMRI, PET, single-photon emission computerized tomography, EEG) that compared areas activated by noxious stimuli between groups of healthy individuals and patients with fibromyalgia (6). Several regions were identified that were differentially activated (Figure 2).
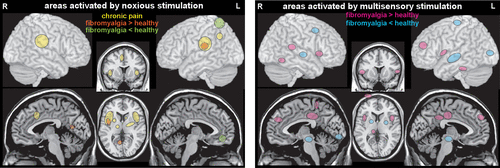
FIGURE 2. Left: A meta-analysis of functional MRI (fMRI) studies of areas activated by noxious stimulation reported very similar patterns for groups of healthy individuals (not illustrated) and of patients with chronic pain conditions (yellow) (5). A meta-analysis that combined studies using multiple functional imaging techniques to compare areas activated by noxious stimulation identified areas that were more (orange) or less (green) activated in fibromyalgia compared to healthy groups (6). Right: A recent study utilized machine learning based on fMRI responses to sensory stimuli to identify a pattern of activation differences that accurately distinguished patients with fibromyalgia from healthy individuals (7). The derived classifier map comprised enhanced activations (pink) in heteromodal and self-referential regions and reduced activations (blue) in primary and secondary visual and auditory regions, lateral prefrontal cortex, cerebellum, diencephalon and midbrain.
Although the regions commonly activated by painful stimuli have been historically referred to as the pain matrix, there is considerable evidence that these areas cannot be considered pain-specific (3, 49–52). This was most clearly demonstrated in an fMRI study in which areas activated by noxious stimuli were compared between healthy individuals and patients with congenital insensitivity to pain (49). The same set of brain regions was activated in both groups. The areas matched the previously identified signature for pain. As noted by the authors, their results confirm that activations indicate high salience rather than pain (49). The presence in fibromyalgia of elevated sensitivity to other types of sensory input (e.g., everyday sounds, sights, and odors) supports the recently proposed generalized or global state of sensory amplification as a result of “top-down” central mechanisms (20). A recent study utilized machine learning based on fMRI responses to sensory stimuli to identify a brain signature for fibromyalgia (Figure 2) (7). The derived multisensory evoked classifier map had high accuracy in correctly identifying fibromyalgia from healthy controls (sensitivity 84%, specificity 94%). Fibromyalgia was characterized by increased activation in heteromodal and self-referential regions and reduced activation in primary/secondary visual and auditory regions, lateral prefrontal, cerebellum, diencephalon and midbrain (7). As noted in a recent review, although multivariate methods are quite useful in the research setting, they are much less likely to become useful in the clinical context (3).
The other major fMRI approach to identifying neurobiological differences associated with fibromyalgia does not require stimulation of any type. Instead, fMRI acquired in a resting state is used to assess spontaneous changes in activation (3, 8, 9). One use of resting state fMRI is evaluation of functional connectivity between brain regions. The most common approach is to use an ROI as a seed and identify voxels throughout the brain in which spontaneous changes in local signal intensity are highly similar to the seed (e.g., highly correlated areas are considered to be functionally connected). Although multiple studies have reported significant differences between fibromyalgia and healthy groups, there is relatively little consistency across studies. This is likely a result of differences in methodology (e.g., inclusion/exclusion criteria, which areas were utilized as seed ROIs) and the small numbers of study subjects examined (53). One research group recently pooled resting state fMRI data from their previous studies and used graph theoretical methods to assess functional connectivity between 264 ROIs (54). The groups had similar global network metrics (i.e., global efficiency, clustering coefficient, average path length, and modularity), indicating similar overall network efficiency and structure. The groups differed, however, in which areas were most highly connected (hubs). Hubs in the fibromyalgia group were predominately located in the salience (insula), somatomotor (primary sensory and motor cortices, supplementary motor area) and auditory (superior temporal cortex) networks. Hubs in the healthy group were predominately located in the default mode, frontoparietal and visual networks. Within the fibromyalgia group, hub structure was influenced by the level of clinical pain (i.e., high, medium, and low). The most interconnected hubs (rich club) in the high pain fibromyalgia subgroup were in areas identified as hubs only in fibromyalgia. In contrast, rich club hubs in the low pain fibromyalgia group resembled the healthy pattern. As noted by the authors, the identified differences in hub topology support the presence of pain-related functional reorganization in fibromyalgia (54). The specific differences must be considered preliminary, as their validation study replicated some findings but not others.
From the clinical perspective, the most intriguing may be the few longitudinal studies in fibromyalgia patients that incorporated both resting state fMRI functional connectivity metrics and clinical measures before and after treatment (8, 9). Studies using a variety of interventions (medications, acupuncture, exercise therapy, transcranial direct current stimulation, and cognitive-behavioral therapy) have demonstrated treatment-related changes in functional connectivity, most of which correlated with improvement in clinical metrics (Figure 3) (10, 11, 55–58). One group of investigators also explored the potential of pretreatment functional connectivity to predict treatment response (Figure 3) (11, 56, 57). Although these studies indicate potential for identifying resting state fMRI-based biomarkers for tracking treatment-induced normalization of function and for treatment selection, a great deal more will be required for clinical validation (3, 53, 59).
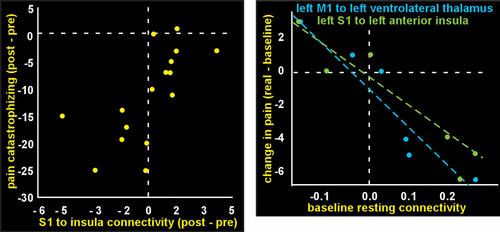
FIGURE 3. AND COVER. From the clinical perspective, the most intriguing may be the longitudinal studies in patients with fibromyalgia that incorporated both resting state fMRI functional connectivity metrics and clinical measures before and after treatment (8, 9). Far left: Studies using a variety of interventions have demonstrated treatment-related changes in functional connectivity, most of which correlated with improvement of clinical metrics. Graphed are one set of results from a study comparing cognitive-behavioral therapy (CBT) to fibromyalgia-specific education (10). Decreases in functional connectivity between the seed (yellow outline on cover) in primary somatosensory cortex (S1) and anterior/middle insula (yellow circle on cover) were greater in the CBT group, and this correlated with decreases in pain catastrophizing. Left: One group has also explored the potential of pre-treatment functional connectivity to predict treatment response. In a study comparing sham and real transcranial direct current stimulation (tDCS) of primary motor cortex (M1), several connections were identified for which stronger baseline functional connectivity predicted greater tDCS induced reduction in pain (11). Graphed are data points from some subjects that could be matched across figures by their unique change in pain, color coded by the connection assessed (seed regions are outlines and target regions are circles on cover).
Conclusions
There are several limitations to be considered in understanding the neurobiological indicators of altered centralized pain processing in fibromyalgia. The presence of central sensitization is widely cited in the literature. However, differences across studies in study design, subject selection and neuroimaging techniques make it difficult to compare results. Areas of the brain implicated have some commonalities across studies, but there is relatively little evidence of a neurobiological signature associated with fibromyalgia specifically or central sensitization more broadly. The evidence to date suggests that fibromyalgia is likely a heterogeneous condition with multiple etiologies.
1 : Gray matter atrophy within the default mode network of fibromyalgia: a meta-analysis of voxel-based morphometry studies. BioMed Res Int 2016; 2016:7296125Crossref, Medline, Google Scholar
2 : Gray matter abnormalities associated with fibromyalgia: a meta-analysis of voxel-based morphometric studies. Semin Arthritis Rheum 2016; 46:330–337Crossref, Medline, Google Scholar
3 : Neuroimaging of pain: human evidence and clinical relevance of central nervous system processes and modulation. Anesthesiology 2018; 128:1241–1254Crossref, Medline, Google Scholar
4 : Histological underpinnings of grey matter changes in fibromyalgia investigated using multimodal brain imaging. J Neurosci 2017; 37:1090–1101Crossref, Medline, Google Scholar
5 : Functional reorganisation in chronic pain and neural correlates of pain sensitisation: a coordinate based meta-analysis of 266 cutaneous pain fMRI studies. Neurosci Biobehav Rev 2016; 68:120–133Crossref, Medline, Google Scholar
6 : Coordinate-based (ALE) meta-analysis of brain activation in patients with fibromyalgia. Hum Brain Mapp 2016; 37:1749–1758Crossref, Medline, Google Scholar
7 : Towards a neurophysiological signature for fibromyalgia. Pain 2017; 158:34–47Crossref, Medline, Google Scholar
8 : What has functional connectivity and chemical neuroimaging in fibromyalgia taught us about the mechanisms and management of centralized pain? Arthritis Res Ther 2014; 16:425Crossref, Medline, Google Scholar
9 : Functional connectivity alterations: novel therapy and future implications in chronic pain management. Pain Physician 2018; 21:E207–E214Crossref, Medline, Google Scholar
10 : Effects of cognitive-behavioral therapy (CBT) on brain connectivity supporting catastrophizing in fibromyalgia. Clin J Pain 2017; 33:215–221Medline, Google Scholar
11 : Changes in resting state functional connectivity after repetitive transcranial direct current stimulation applied to motor cortex in fibromyalgia patients. Arthritis Res Ther 2016; 18:40Crossref, Medline, Google Scholar
12 : Facts and myths pertaining to fibromyalgia. Dialogues Clin Neurosci 2018; 20:53–62Medline, Google Scholar
13 : Prevalence of fibromyalgia in general population and patients, a systematic review and meta-analysis. Rheumatol Int 2017; 37:1527–1539Crossref, Medline, Google Scholar
14 : Gender-stratified prevalence of psychiatric and pain diagnoses in a primary care patient sample with fibromyalgia. Pain Med 2019; pnz084Medline, Google Scholar
15 : Fibromyalgia: a critical and comprehensive review. Clin Rev Allergy Immunol 2015; 49:100–151Crossref, Medline, Google Scholar
16 : Central sensitization in fibromyalgia? A systematic review on structural and functional brain MRI. Semin Arthritis Rheum 2014; 44:68–75Crossref, Medline, Google Scholar
17 : Neurobiology of fibromyalgia and chronic widespread pain. Neuroscience 2016; 338:114–129Crossref, Medline, Google Scholar
18 : Fibromyalgia syndrome: a somatoform disorder? Eur J Pain 2014; 18:1052–1059Crossref, Medline, Google Scholar
19 : Controversies and challenges in fibromyalgia: a review and a proposal. Ther Adv Musculoskelet Dis 2017; 9:115–127Crossref, Medline, Google Scholar
20 : The neurobiology of central sensitization. J Appl Behav Res 2018; 23:e12137Crossref, Google Scholar
21 : Multidimensional diagnostic criteria for chronic pain: introduction to the ACTTION-American Pain Society Pain Taxonomy (AAPT). J Pain 2016; 17(Suppl):T1–T9Crossref, Medline, Google Scholar
22 : Chronic pain as a symptom or a disease: the IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 2019; 160:19–27Crossref, Medline, Google Scholar
23 : AAPT diagnostic criteria for fibromyalgia. J Pain 2018; S1526-5900(18)30832-0Medline, Google Scholar
24 : Fibromyalgia and related conditions. Mayo Clin Proc 2015; 90:680–692Crossref, Medline, Google Scholar
25 : Subjective experiences and sensitivities in women with fibromyalgia: a quantitative and comparative study. Pain Res Manag 2018; 2018:8269564Crossref, Medline, Google Scholar
26 : A systematic review of precipitating physical and psychological traumatic events in the development of fibromyalgia. Semin Arthritis Rheum 2018; 48:121–133Crossref, Medline, Google Scholar
27 : Fibromyalgia: genetics and epigenetics insights may provide the basis for the development of diagnostic biomarkers. Mol Pain 2019; 15:1744806918819944Crossref, Google Scholar
28 : The role of psychosocial processes in the development and maintenance of chronic pain. J Pain 2016; 17(Suppl):T70–T92Crossref, Medline, Google Scholar
29
30 : Symptoms, the nature of fibromyalgia, and diagnostic and statistical manual 5 (DSM-5) defined mental illness in patients with rheumatoid arthritis and fibromyalgia. PLoS One 2014; 9:e88740Crossref, Medline, Google Scholar
31 : Construct validity and clinical utility of current research criteria of DSM-5 somatic symptom disorder diagnosis in patients with fibromyalgia syndrome. J Psychosom Res 2015; 78:546–552Crossref, Medline, Google Scholar
32 : The management of fibromyalgia from a psychosomatic perspective: an overview. Int Rev Psychiatry 2017; 29:473–488Crossref, Medline, Google Scholar
33 : Management of somatic symptom disorder. Dialogues Clin Neurosci 2018; 20:23–31Medline, Google Scholar
34 : The complexities of fibromyalgia and its comorbidities. Curr Opin Rheumatol 2018; 30:94–100Crossref, Medline, Google Scholar
35 : Reframing chronic pain as a disease, not a symptom: rationale and implications for pain management. Postgrad Med 2019; 131:185–198Crossref, Medline, Google Scholar
36 : Comorbidity between pain and mental illness: evidence of a bidirectional relationship. Eur J Pain 2018; 22:1304–1311Crossref, Medline, Google Scholar
37 : Phenotypic features of central sensitization. J Appl Biobehav Res 2018; 23:e12135Crossref, Medline, Google Scholar
38 : Pain amplification: a persepctive on the how, why, when, and where of central sensitization. J Appl Behav Res 2018; 23:e12124Crossref, Google Scholar
39 : Central sensitization: a brief overview. J Appl Behav Res 2018; 23:e12138Crossref, Google Scholar
40 : Central sensitization: implications for the diagnosis and treatment of pain. Pain 2011; 152(Suppl):S2–S15Crossref, Medline, Google Scholar
41 : Defective endogenous pain modulation in fibromyalgia: a meta-analysis of temporal summation and conditioned pain modulation paradigms. J Pain 2018; 19:819–836Crossref, Medline, Google Scholar
42 : Melatonin analgesia is associated with improvement of the descending endogenous pain-modulating system in fibromyalgia: a phase II, randomized, double-dummy, controlled trial. BMC Pharmacol Toxicol 2014; 15:40Crossref, Medline, Google Scholar
43 : A pilot study investigating whether quantitative sensory testing alters after treatment in patients with fibromyalgia. Br J Pain 2018; 12:250–256Crossref, Medline, Google Scholar
44 : Mindful yoga pilot study shows modulation of abnormal pain processing in fibromyalgia Patients. Int J Yoga Therap 2016; 26:93–100Crossref, Medline, Google Scholar
45 : Neuroimaging of central sensitivity syndromes: key insights from the scientific literature. Curr Rheumatol Rev 2016; 12:55–87Crossref, Medline, Google Scholar
46 : Gray matter changes following cognitive behavioral therapy for patients with comorbid fibromyalgia and insomnia: a pilot study. J Clin Sleep Med 2018; 14:1595–1603Crossref, Medline, Google Scholar
47 : Brain glial activation in fibromyalgia - A multi-site positron emission tomography investigation. Brain Behav Immun 2019; 75:72–83Crossref, Medline, Google Scholar
48 : Brain activations during pain: a neuroimaging meta-analysis of patients with pain and healthy controls. Pain 2016; 157:1279–1286Crossref, Medline, Google Scholar
49 : The “pain matrix” in pain-free individuals. JAMA Neurol 2016; 73:755–756Crossref, Medline, Google Scholar
50 : Pain perception: multiple matrices or one? JAMA Neurol 2016; 73:628–630Crossref, Medline, Google Scholar
51 : Recent advances in objectifying pain using neuroimaging techniques. J Neurophysiol 2018; 120:387–390Crossref, Medline, Google Scholar
52 : Modeling pain using fMRI: from regions to biomarkers. Neurosci Bull 2018; 34:208–215Crossref, Medline, Google Scholar
53 : Pain neuroimaging in humans: a primer for beginners and non-imagers. J Pain 2018; 19:961.e1–961.e21Crossref, Google Scholar
54 : Functional and neurochemical disruptions of brain hub topology in chronic pain. Pain 2019; 160:973–983Crossref, Medline, Google Scholar
55 : Decreased intrinsic brain connectivity is associated with reduced clinical pain in fibromyalgia. Arthritis Rheum 2012; 64:2398–2403Crossref, Medline, Google Scholar
56 : Pregabalin rectifies aberrant brain chemistry, connectivity, and functional response in chronic pain patients. Anesthesiology 2013; 119:1453–1464Crossref, Medline, Google Scholar
57 : Resting state connectivity correlates with drug and placebo response in fibromyalgia patients. Neuroimage Clin 2014; 6:252–261Crossref, Medline, Google Scholar
58 : Normalization of aberrant resting state functional connectivity in fibromyalgia patients following a three month physical exercise therapy. Neuroimage Clin 2015; 9:134–139Crossref, Medline, Google Scholar
59 : Deconstructing biomarkers for chronic pain: context- and hypothesis-dependent biomarker types in relation to chronic pain. Pain 2019; 160(Suppl 1):S37–S48Crossref, Medline, Google Scholar



